
CMIC: Japan’s leading CRO focuses on the future of clinical trials with innovation and digital solutions
In last year’s feature, we spotlighted CMIC Group as Japan’s leading CRO, transforming clinical trials by streamlining processes and bridging gaps between pharmaceutical companies and Japanese medical institutions. Fast forward to today, CMIC continues to redefine the landscape of drug development, pushing boundaries with its innovative approaches and expanding capabilities. Under the leadership of Kazuo Nakamura, CMIC Group continues to grow its Site Management Organization (SMO) bolstering its support services for clinical trials with a robust network of over 4,000 medical institutions. CMIC’s comprehensive services, including feasibility surveys and regulatory oversight, ensure high-quality trial operations. Meanwhile their specialist Clinical Research Coordinators (CRCs) and Site Management Associates (SMAs) play key roles in patient engagement and recruitment.
This year, CMIC is further advancing its mission with cutting-edge digital health solutions. Platforms like harmo and nanacara are enhancing patient-centric care by integrating comprehensive health data and improving recruitment and engagement processes. Looking ahead to 2025, CMIC’s focus remains on leveraging these digital tools and advanced technologies to support innovative drug development and foster patient well-being. Staying true to the Japanese concept of IKIGAI – helping individuals live life to the fullest – remains CMIC’s most important core value.
Interviewer:
- John Carroll, Founder & Editor, Endpoints News
Interviewees:
- Kazuo Nakamura, Ph.D. – Founder & CEO, CMIC Group
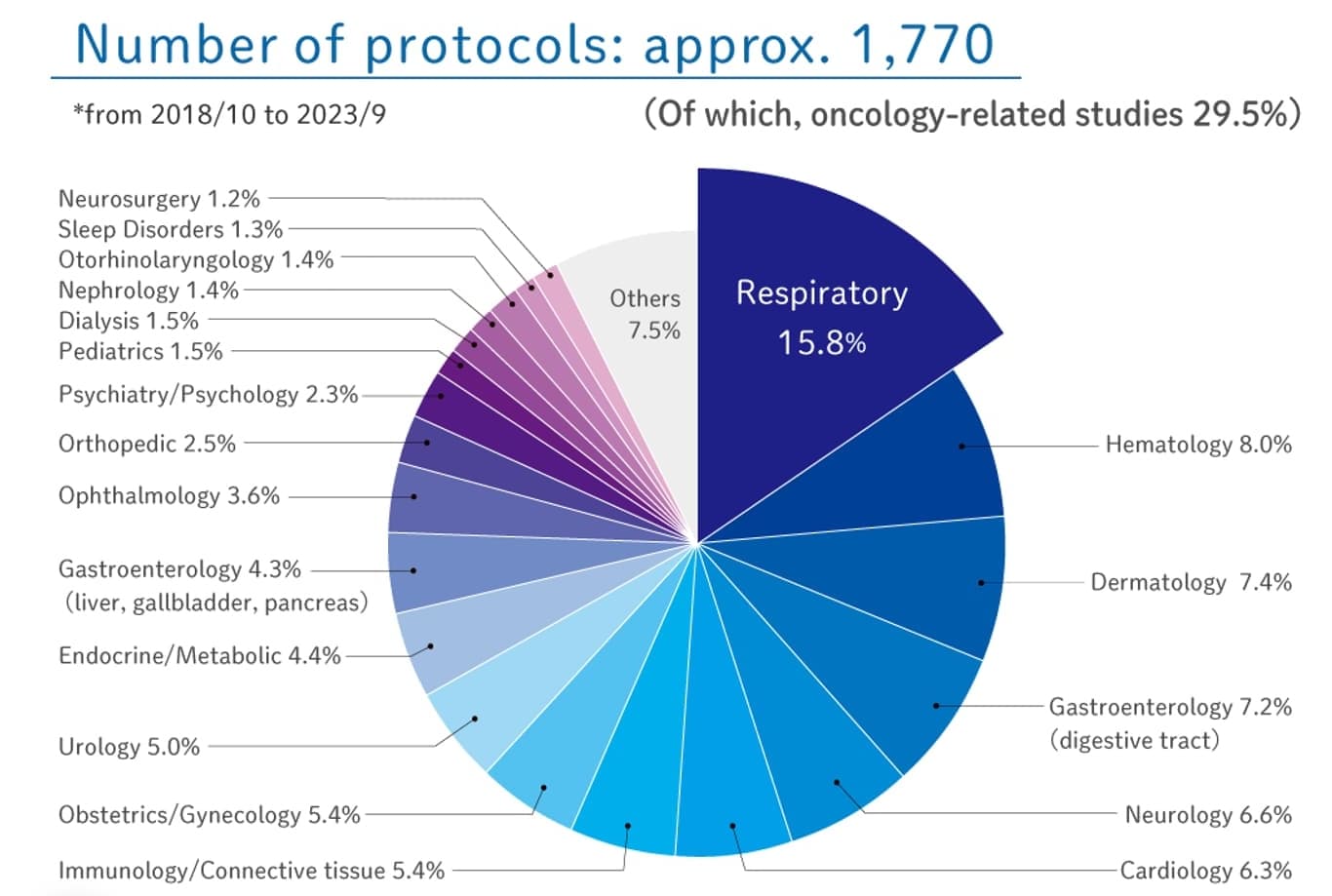
CMIC’s Site Management Organization (SMO)
Endpoints: Can you describe the range of clinical trial support services that CMIC Group offers and how these services benefit clinical trial sponsors?

All of the news, delivered with full-text to your inbox. For professionals discovering, developing, and marketing biopharmaceutical drugs.
Kazuo Nakamura: CMIC Group is APAC’s leading clinical CRO. We are also Japan’s first SMO with over 25 years of experience accelerating clinical trials. We offer comprehensive support to sponsors, facilitating collaboration between pharmaceutical companies, CROs, and Japanese medical institutions. As an SMO, we coordinate clinical trial logistics, including feasibility surveys that leverage our medical institution network, contract procedures, and clinical staff support. We also oversee Institutional Review Boards (IRBs) and essential trial documents. In Japan, where trial agreements are between sponsors and medical institutions, CMIC’s team of Clinical Research Coordinators (CRCs) and Site Management Associates (SMAs) ensures trials are conducted efficiently and to a high standard.
CRCs improve the quality of clinical trials by managing key areas such as patient eligibility, informed consent support, hospital visits, testing schedules and other clinical trial materials such as preparing Case Report Forms (CRFs). To facilitate smooth clinical trials, they provide additional support to Clinical Research Associates (CRAs) and assist medical institutions with audits/regulatory inspections.
SMAs are responsible for a wide range of external relations and management tasks to support the medical institutions on a daily basis. This includes conducting feasibility studies and site preparation for clinical trials, handling invoices and document creation, providing support for IRB submissions and reviews, and assisting with audits and regulatory inspections.
Endpoints: How does CMIC Group ensure successful patient engagement and retention in clinical trials through its unique SMO services?
Kazuo Nakamura: CMIC Group ensures successful patient engagement and retention through its SMO services by guiding sponsors to high-quality trial sites and conducting feasibility studies to ensure success in the clinical setting. Our CRCs maintain daily communication with sites, addressing concerns and reducing dropout rates. We leverage a nationwide network of SMO-affiliated sites and partner companies, streamlining patient referrals and using targeted recruitment strategies. CMIC also partners with patient advocacy groups for outreach and education. In a NASH trial, we swiftly enrolled 2,000 patients through advocacy and advertising, while COVID-19 vaccine trials exceeded the 1000-patient target by collaborating with 13 advocacy groups, enhancing recruitment and overall data quality.
Endpoints: What advantages do CMIC Group’s extensive network and experience in diverse therapeutic areas offer to global clinical trial sponsors?
Kazuo Nakamura: CMIC Group’s strength lies in its vast network of 4,000 medical institutions, deep expertise, and comprehensive database, enabling us to recommend the most suitable sites for each therapeutic area. We offer unique patient recruitment strategies and call center services to meet diverse sponsor needs across various disease areas. Collaborating with both global and local pharmaceutical companies, we help navigate differences between Japanese and global guidelines. By connecting sponsors with appropriate medical institutions and managing time-consuming tasks like IRB submissions, CMIC’s SMO services are crucial in supporting CROs and sponsors efficiently, especially at larger clinical sites with heavier workloads.
Digital Health in Japan
Endpoints: As a company heavily invested in digital health, how is CMIC Group leveraging digital technology to enhance its patient-centric solutions for customers?
Kazuo Nakamura: CMIC Group leverages digital health through its patient-centric platforms, harmo and nanacara.
Firstly, harmo is a Patient Health Record (PHR) platform that integrates with Japan’s My Number Portal, pharmacy and hospitals – automatically updating medical records and eliminating manual input. Meanwhile, nanacara, initially focused on epilepsy, helps patients and caregivers manage seizures and medication, while providing tools to monitor daily patient status and improve clinical decision making.
These platforms enhance clinical trials by improving patient recruitment, boosting engagement with interactive features, and enriching data quality. By streamlining patient monitoring and data collection, harmo and nanacara make trials more efficient and patient-friendly.
Endpoints: How is CMIC Group planning to expand the use of innovative technologies to further support patient-centricity and diversity in 2025?
Kazuo Nakamura: In 2025, CMIC Group plans to expand harmo – connecting hospitals, pharmacies, and patients – to address operational challenges in healthcare. Along with existing integration efforts with nancara, harmo will integrate with platforms like therapeutic support apps, life insurance systems, fleet management and public health services, creating synergies across different sectors. Additionally, nanacara, initially focused on epilepsy, will expand into dementia care in Japan and other Asian countries. By collaborating with pharmaceutical companies to address unmet medical needs, CMIC aims to extend nanacara to other disease areas, further supporting patient-centric care and diversity.
Leadership and IKIGAI
Endpoints: How does CMIC Group balance fostering innovation with maintaining a focus on the core value of IKIGAI: “living life to the fullest”?
Kazuo Nakamura: As referenced in last year’s article, IKIGAI, a Japanese concept of living life to the fullest. Research coming out of Harvard has shown that IKIGAI principles are linked to improved health outcomes like optimal blood pressure, cholesterol and blood sugar. This is partly due to the positive impact of strong interpersonal relationships. CMIC Group embraces IKIGAI as a core value, balancing it with innovation to focus on holistic well-being and longevity. While traditional pharmaceuticals treat and prevent diseases, IKIGAI emphasizes a holistic desire to live well. As Japan faces an aging society with a low birth rate, the nation is pioneering efforts to balance these challenges, which is reflected in its advanced healthcare model. CMIC aims to integrate IKIGAI into its pharmaceutical value chain. At the 30th DIA Annual Meeting in Japan – where I will be the chairperson – CMIC will explore how IKIGAI can expand globally, helping people live more fulfilling lives.
Endpoints: Can you provide examples of how CMIC Group’s leadership has successfully integrated IKIGAI into its innovative projects?
Kazuo Nakamura: IKIGAI is central to our digital health initiatives, which go beyond treatment to support well-being by enabling individuals to pursue hobbies and passions despite physical limitations. For example, a passionate golfer with a heart condition can use digital tools such as health parameter monitors and digital therapeutics, in order to continue enjoying the sport with peace of mind. As AI and digitalization evolve, data will transform into actionable insights, empowering people to follow their IKIGAI even with health challenges. While measuring IKIGAI through digital tools is still emerging, these technologies increasingly help people live fuller, more fulfilling lives. Embracing this shift is the healthiest and smartest way forward.
Learn more about CMIC Group’s solutions here.